Õygen – Properties, Occurrence, and Allotropes Explained!

History:
Oxygen, often referred to as Õygen in various contexts, has a fascinating history that dates back to the 18th century. The discovery of oxygen is credited to multiple scientists working independently. In 1771, Carl Wilhelm Scheele, a Swedish pharmacist, discovered a gas he called “fire air” due to its ability to support combustion.
However, Scheele did not publish his findings until after Joseph Priestley, an English chemist, independently discovered the same gas in 1774.
Priestley conducted an experiment in which he heated mercuric oxide (HgO) and collected the gas released, which he found could relight a glowing splint.
Priestley published his findings immediately, which led to him being credited with the discovery.
Antoine Lavoisier, a French chemist, also conducted experiments on the gas and played a crucial role in understanding its properties.
Lavoisier debunked the phlogiston theory, which was the prevalent explanation for combustion and rusting at the time. He demonstrated that combustion.
Respiration were due to a substance that he named “oxygen” from the Greek words “oxys” (acid) and “genes” (producer), reflecting his belief that oxygen was necessary for the formation of acids.
Occurrence and Properties:

Oxygen is the third most abundant element in the universe, following hydrogen and helium. It makes up about 21% of the Earth’s atmosphere by volume and is an essential component of the Earth’s crust, found in water (H2O), minerals, and rocks.
In its elemental form, oxygen exists as a diatomic molecule (O2), which is a colorless, odorless, and tasteless gas under standard conditions. It has a density of about 1.429 grams per liter, making it slightly heavier than air.
Oxygen is highly reactive and readily forms compounds with most elements. Its high electronegativity makes it a strong oxidizing agent. It is essential for the process of aerobic respiration in living organisms, where it acts as the terminal electron acceptor in the electron transport chain, allowing for the production of ATP, the energy currency of cells.
Allotropy:
Oxygen exists in several allotropes, the most common of which are dioxygen (O2) and ozone (O3). Dioxygen is the form that is most familiar to us, as it constitutes the oxygen gas we breathe. It is crucial for life on Earth and is involved in various biological and chemical processes.
Ozone, another allotrope, consists of three oxygen atoms bonded together (O3). It is found in the Earth’s stratosphere, where it forms the ozone layer.
This layer is vital for life on Earth as it absorbs the majority of the sun’s harmful ultraviolet radiation, protecting living organisms from its damaging effects. However, ozone at ground level is a pollutant and can have harmful effects on respiratory health.
A less common allotrope is tetraoxygen (O4), which exists under high-pressure conditions. There is also a cluster form known as oxygen hexafluoride (O6), which has been observed in certain experimental conditions.
Preparative Methods:
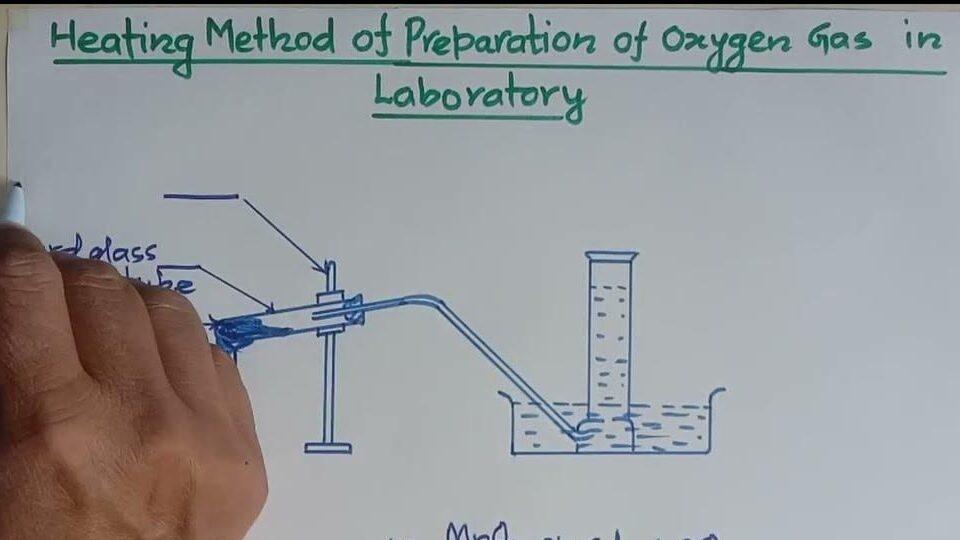
In laboratory settings, oxygen can be prepared through several methods. One common method is the thermal decomposition of potassium chlorate (KClO3).
When heated in the presence of a catalyst such as manganese dioxide (MnO2), potassium chlorate decomposes to produce potassium chloride (KCl) and oxygen gas (O2): 2KClO3→heat,MnO22KCl+3O22KClO_3 \xrightarrow{heat, MnO_2} 2KCl + 3O_22KClO3heat,MnO22KCl+3O2
Another method for preparing oxygen is the electrolysis of water. In this process, an electric current is passed through water, causing it to decompose into oxygen and hydrogen gases: 2H2O→electricity2H2+O22H_2O \xrightarrow{electricity} 2H_2 + O_22H2Oelectricity2H2+O2
Oxygen can also be obtained by heating certain metal oxides, which decompose to release oxygen.
For example, heating mercuric oxide (HgO) results in the formation of mercury and oxygen: 2HgO→heat2Hg+O22HgO \xrightarrow{heat} 2Hg + O_22HgOheat2Hg+O2
Commercial Production and Use:
Commercially, oxygen is primarily produced through the fractional distillation of liquefied air. This process involves cooling air to very low temperatures to liquefy it, and then slowly warming it up.
As the temperature rises, different components of air boil off at different temperatures. Nitrogen, which has a lower boiling point, evaporates first, leaving behind liquid oxygen, which can then be collected.
Another commercial method is the pressure swing adsorption (PSA) process. In this method, air is passed through a bed of zeolite molecular sieves that selectively adsorb nitrogen, leaving behind a stream of enriched oxygen.
Oxygen has a wide range of industrial applications. In the medical field, oxygen therapy is essential for patients with respiratory conditions, and oxygen is a critical component of life support systems. In metallurgy, oxygen is used in the cutting and welding of metals.
The oxy-acetylene torch, which burns a mixture of oxygen and acetylene, produces a flame hot enough to melt steel.
In the chemical industry, oxygen is used to produce synthetic gas (syngas), a mixture of hydrogen and carbon monoxide, which serves as a precursor for various chemicals and fuels.
Oxygen is also used in wastewater treatment processes to promote the aerobic breakdown of organic matter by bacteria, improving water quality. Additionally, it is used in the production of glass and steel, where it helps remove impurities and improve product quality.
Chemical Properties and Reactions:
Being a very reactive element, oxygen can create oxides with nearly every other element. Its reactions can be broadly classified into combustion, oxidation, and reduction processes.
In combustion reactions, oxygen reacts with a fuel (usually a hydrocarbon) to produce carbon dioxide, water, and energy. This exothermic reaction is the basis for many everyday processes, such as burning wood, gasoline in car engines, and natural gas in heating systems: CH4+2O2→CO2+2H2O+energyCH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + energyCH4+2O2→CO2+2H2O+energy
In oxidation reactions, oxygen adds to other substances, often resulting in the formation of oxides. For example, iron reacts with oxygen to form iron oxide (rust): 4Fe+3O2→2Fe2O34Fe + 3O_2 \rightarrow 2Fe_2O_34Fe+3O2→2Fe2O3
Reduction reactions involve the gain of electrons, where oxygen is typically reduced by other substances. In biological systems, oxygen is reduced to water during cellular respiration: O2+4H++4e−→2H2OO_2 + 4H^+ + 4e^- \rightarrow 2H_2OO2+4H++4e−→2H2O
Oxygen’s role in redox reactions is fundamental to numerous biochemical processes essential for life. In the human body, oxygen is transported by hemoglobin in red blood cells from the lungs to tissues, where it is used in cellular respiration to produce ATP.
FAQs About Õygen:
1. Who discovered oxygen and when?
Joseph Priestley discovered oxygen in 1774, and Carl Wilhelm Scheele made the discovery on his own in 1771. Antoine Lavoisier later named the element and explained its properties.
2. What are the most common forms of oxygen?
The most common forms of oxygen are dioxygen (O2) and ozone (O3). Dioxygen is the form we breathe, while ozone forms a protective layer in the stratosphere.
3. How is oxygen commercially produced?
Oxygen is primarily produced through fractional distillation of liquefied air and the pressure swing adsorption (PSA) process.
4. What are some industrial uses of oxygen?
Oxygen is used in medical therapies, metal cutting and welding, chemical production, wastewater treatment, and in the manufacture of glass and steel.
5. What role does oxygen play in biological systems?
In biological systems, oxygen is crucial for aerobic respiration, where it acts as the final electron acceptor in the electron transport chain, producing ATP, the energy currency of cells.
Conclusion
Oxygen, also known as Õygen, is a vital element with a rich history and significant impact on life and industry. Discovered in the 18th century, it is essential for respiration, industrial processes, and environmental protection. Its various forms and reactivity make it indispensable in both biological and chemical contexts. Understanding oxygen’s properties and applications highlights its crucial role in our world.



Leave a Comment